Blog
Myotilin
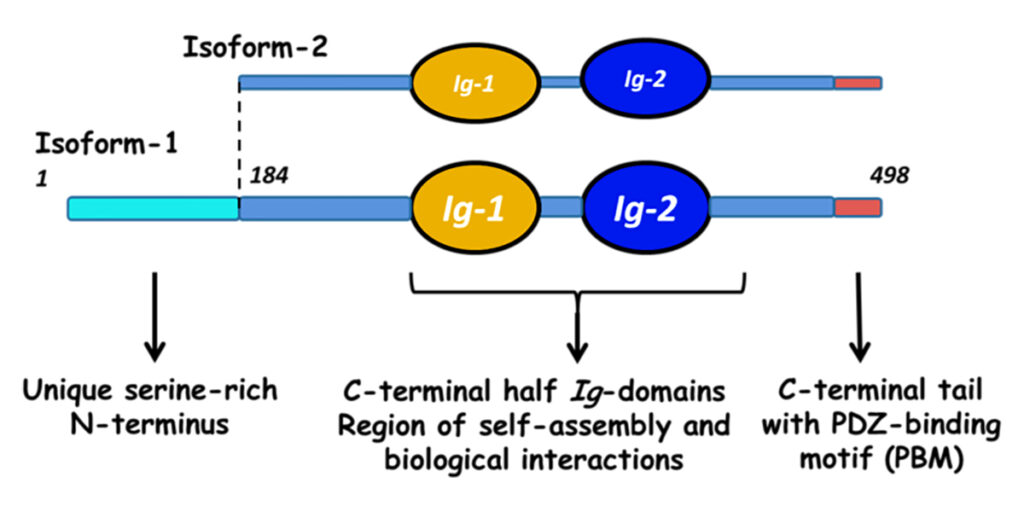
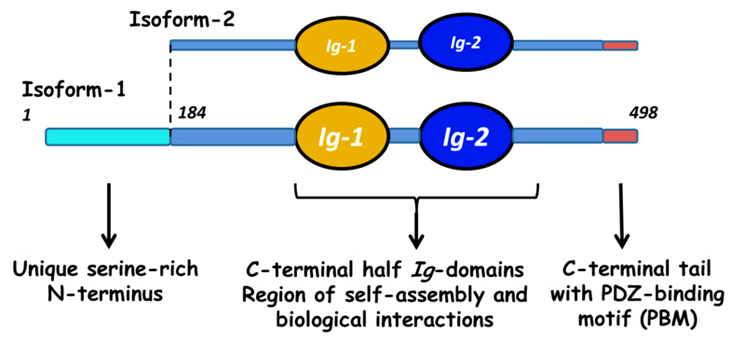
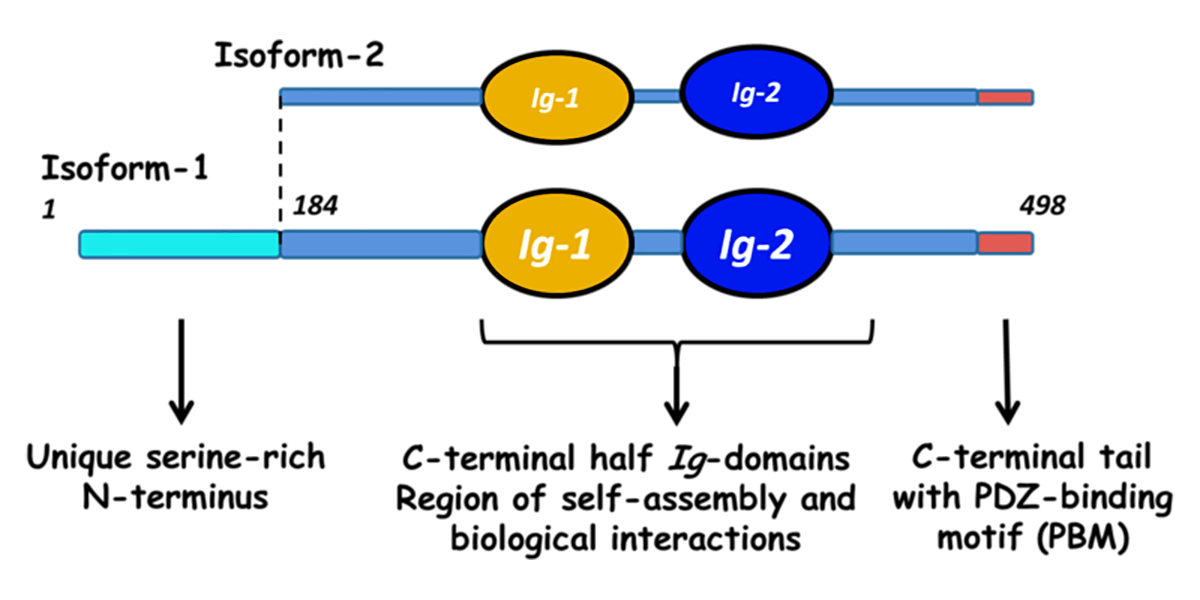
Myotilin is a muscle protein encoded by the MYOT gene. Myotilin is a structural protein of muscle that localizes to the Z discs. Myotilin contains two Immunoglobulin-like (Ig-like) domains similar to those of palladin, myopalladin, titin, and other proteins. The amino-terminal portion of myotilin contains a large number of serine residues and is not similar to any other known protein.
Human patients with Limb-Girdle Muscular Dystrophy (LGMD) or Myofibrillar Myopathy (MFM) have been found to have mutations in MYOT. Both LGMD and MFM are disease states associated with mutations in a number of different genes. A substantial fraction of human patients with LGMD or MFM do not receive a molecular diagnosis, suggesting that there are undiscovered genetic variants responsible for some of these cases.
Human patients with LGMD that have mutations in MYOT are diagnosed with Limb-Girdle Muscular Dystrophy 1A (LGMD1A), while human patients with MFM that have mutations in MYOT are diagnosed with Myofibrillar Myopathy 3 (MFM3). Pathogenic mutations in MYOT are dominant missense alleles. Known pathogenic alleles include S55F, T57I, S60C, S60F, and S95I [1, 2, 3].
There is a substantial difference in the age of onset and the symptoms of human patients with MYOT mutations, even among patients carrying the same MYOT allele. This suggests that genetic background, that is, mutations in other genes, has an influence on the symptoms seen in LGMD1A and MFM3. Myotilin has been shown to interact with alpha-actin 1 (ACTA1), alpha-actinin 1 (ACTN1), filamin A (FLNA), filamin B (FLNB), filamin C (FLNC), and myozenin 2 (MYOZ2) [1, 4, 5, 6, 7].
Because it is difficult to obtain human tissue samples and because human patients with the same MYOT alleles show clinical variability, studies of the effects of mutations in MYOT have been carried out in mice. Transgenic mice expressing the wild-type human MYOT gene or MYOT-T57I were generated and compared [8]. While mice expressing the wild-type human MYOT gene are normal, mice expressing MYOT-T57I show a progressive myofibrillar pathology including Z-disc streaming and the development of aggregates of Z-disc proteins including myotilin, desmin, alpha-actinin, and filamin C. The phenotype of transgenic mice expressing human MYOT-T57I resembles that of human patients with LGMD1A and MFM3.
In contrast, targeted deletion of the MYOT gene in mice has been used to generate mice entirely lacking myotilin [9]. These mice develop normally and show no abnormalities of muscle structure or function [9]. Therefore, the incorporation of myotilin bearing a dominant missense allele (MYOT-T57I) into muscle is more damaging than the complete loss of myotilin.
EquiSeq is currently investigating the effects of MYOT-S232P (P2), a missense allele of MYOT that is common in some horse breeds that appears to be associated with Polysaccharide Storage Myopathy type 2 (PSSM2). The test for MYOT-S232P (P2) is currently part of EquiSeq’s Myopathy Panel, but the assertion that MYOT-S232P (P2) is associated with inherited myopathy in horses has not yet been made in a peer-reviewed scientific publication.
Citations
[1] Hauser, MA et al. (2000). “Myotilin is mutated in limb girdle muscular dystrophy 1A.” Hum Mol Genet. 9(14):2141-2147. PMID: 10958653.
[2] Hauser, MA et al. (2002). “Myotilin mutation found in second pedigree with LGMD1A.” Am J Hum Genet. 71(6):1428-1432. PMID: 12428213.
[3] Selcan, D and Engel, AG (2004). “Mutations in myotilin cause myofibrillar myopathy.” Neurolog. 62(8):1363-71. PMID: 15111675.
[4] Salmikangas, P et al. (1999). “Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy.” Hum Mol Genet. 8(7):1329-1336. PMID: 10369880.
[5] van der Ven, PF et al. (2000). “Indications for a novel muscular dystrophy pathway. gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin.” J Cell Biol. 151(2):235-48. PMID: 11038172.
[6] Salmikangas, P et al. (2003). “Myotilin, the limb-girdle muscular dystrophy 1A (LGMD1A) protein, cross-links actin filaments and controls sarcomere assembly.” Hum Mol Genet. 12(2):189-203. PMID: 12499399.
[7] Gontier, Y et al. (2005). “The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins.” J Cell Sci. 118(Pt 16):3739-3749. PMID: 16076904.
[8] Garvey, SM et al. (2006). “Transgenic mice expressing the myotilin T57I mutation unite the pathology associated with LGMD1A and MFM.” Hum Mol Genet. 15(15):2348-62. PMID: 16801328.
[9] Moza, M et al. (2007). “Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice.” Mol Cell Biol. 27(1):244-52. PMID: 17074808.
Share this post
From the blog
The latest industry news, interviews, technologies, and resources.